Abstract
Introduction: Mantle cell lymphoma (MCL) is usually an aggressive B-cell lymphoma subtype characterized by frequent relapses and is still considered to be incurable. Ibrutinib (iBTK), a first-in-class inhibitor of Bruton′s tyrosine kinase, demonstrated promising outcomes in heavily pretreated MCL patients in several prospective trials. But only limited data on its effect is available in real-world population.
Methods: We performed an analysis of 77 MCL patients (pts) from five Czech university centers diagnosed from 11/1997 to 12/2019. iBTK was initiated no later than 7/2020. The database was locked 7/2021. Bone marrow (BM) was examined by immunohistochemistry and/or flowcytometry at time of iBTK initiation. Overall and progression free survival (OS, PFS) were calculated from the beginning of iBTK therapy. Duration of response (DoR) to iBTK was calculated from response to relapse/progression/death.
Results: The median age at diagnosis was 68 (40-81) years. Eighty percent of pts had advanced disease (III and IV). MIPI score was low, intermediate and high in 15.3%, 20.3% and 64.4% pts, respectively. BM involvement had 27 (56.3%) of 48 evaluated pts. Frontline regimens used were as follows: R-CHOP/R-CHOP-like in 54.5%, intensive R-HDAC-containing in 32.5% and non-anthracycline regimen in 13.0% pts. Autologous stem cell transplant consolidation had 24.7% pts. Rituximab maintenance was administered in 53.2% pts. Sixty-one percent of pts had relapse/progression of disease within 24 months (POD24). Median of treatment lines before iBTK was 2 (1-8). The overall response rate to iBTK by PET/CT was 66% with 30% complete remissions.
After median follow-up of 12.6 months, 26 (33.8%) pts are alive. Median PFS and median OS were 7.9 months (95% CI 1.1-65.6) and 12.4 months (95% CI 1.2-80.0), respectively. Median DoR was 8.8 months (95% CI 0.7-64.1). Pts with 1 line of therapy before iBTK experienced significantly superior OS compared to those with 2 or more previous lines (2-year OS [2-y OS] 66.0% vs 37.4%, p=0.03), but there was no difference in PFS (p=0.46) and DoR (p=0.83). We found a trend toward improved OS in patients without POD24 (2-y OS 51.1% vs 41.0%, p=0.08), but no difference neither in PFS (p=0.97), nor DoR (p=0.16). Survival analysis according to BM involvement status (BM +/-) showed 2-fold lower risk of relapse/progression (HR=1.81, p=0.06) and death (HR=2.18, p=0.02) in BM+ pts. No difference in DoR was found (p=0.72).
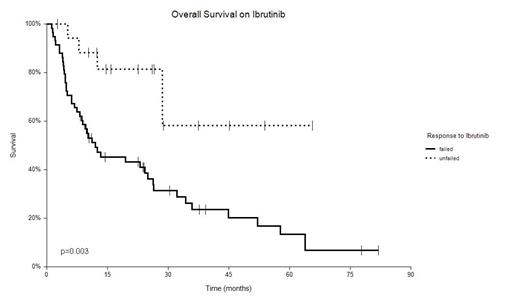
Pts with iBTK failure had almost 4-fold higher risk of death (HR 3.6, p=0.003) [Fig. 1].
Conclusions: Our data confirm promising efficiency of ibrutinib also in heavily pretreated unselected R/R MCL patients but iBTK failure portends a dismal outcomes. Surprisingly, BM involvement at time of iBTK initiation seems to have favorable impact on prognosis and requires further investigation.
Acknowledgement: Supported by IGA_LF_2021_001, MZ ČR - RVO (FNOL, 00098892), PROGRES Q40/08 (FN HK), AZV NU21-03-00386
Belada: Genmab: Research Funding. Trněný: Portola: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Amgen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; 1st Faculty of Medicine, Charles University, General Hospital in Prague: Current Employment; Celgene: Consultancy; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; AstraZeneca: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal